Our value proposition
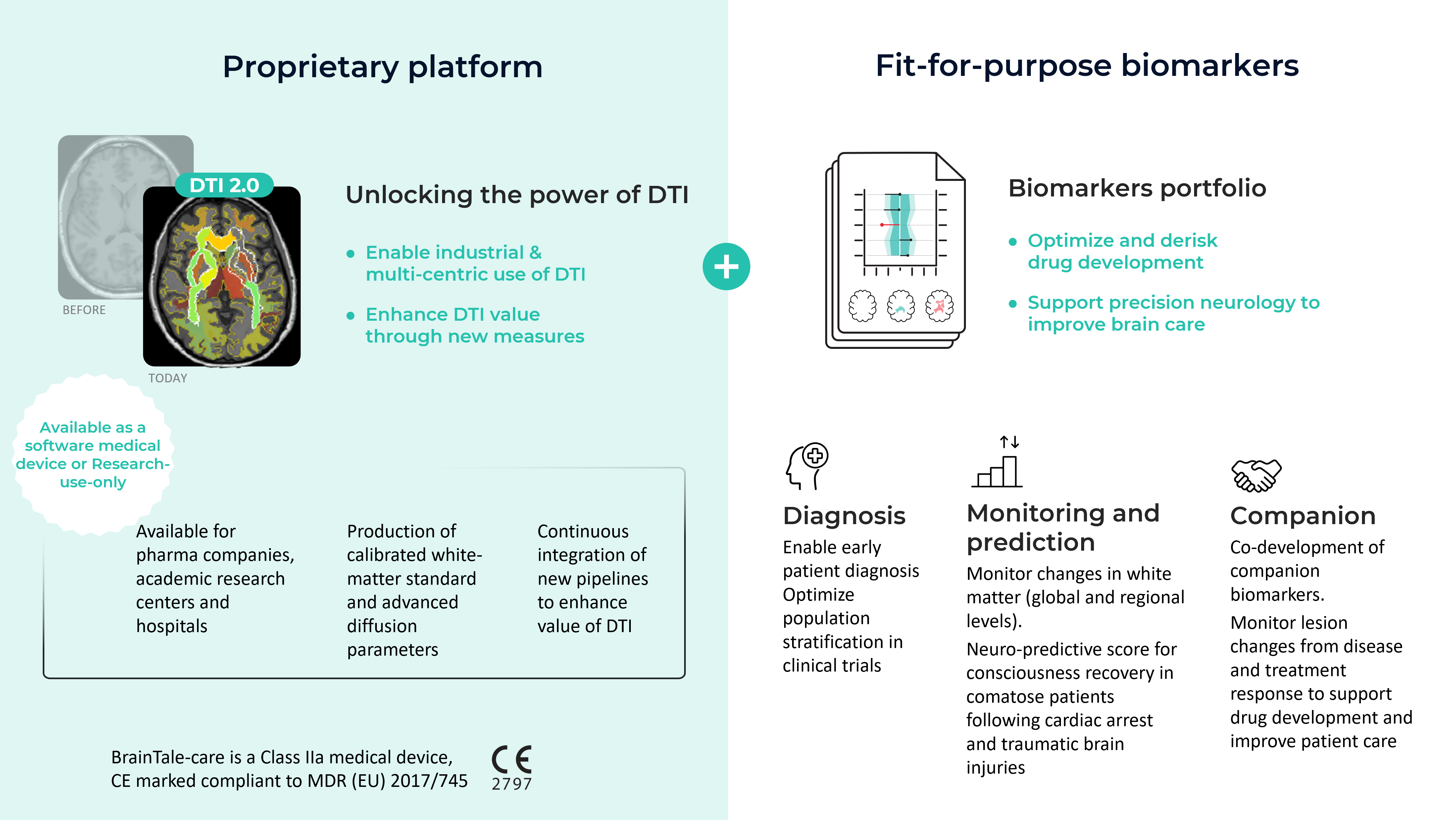
BrainTale offers reliable, reproducible, quantitative and calibrated white-matter measures derived from DTI imaging through its platform brainTale-care and modules brainScore-coma and brainQuant.
BrainTale technology enables rapid and robust brain assessment through the production of calibrated white matter diffusion parameters. These parameters are obtained through post-processing of diffusion MRI, a sequence available on all MRI scanners.
Calibrated brain white matter parameters processed by BrainTale enable precise quantification of white matter alterations – such as demyelination or axonal degeneration – to facilitate diagnosis, monitoring and prognosis of neurological conditions at individual level.
BrainTale technology enables the use of these measures in multi-centric clinical trials as well as to support personalized brain care for patients in care settings, through braintale-care software.
brainTale-care is a clinically validated software medical device available in SaaS (Software as a Service) mode for any physician with access to a diffusion MRI.
Key features of our brainTale-care platform
CALIBRATED
Correct acquisition biais from
scanner and center
STANDARDIZED
Compare with healthy subjects
data to establish statistical standards
QUALITY CONTROLED
Systematic check for data and process quality
- High robustness: Using our proprietary calibration and standardization process, brainTale-care provides reliable and reproducible biomarkers with high test-retest repeatability and inter-center reproducibility.
- Regulatory approved and GDPR compliant: CE marked Class IIa medical device
- Secure of our servers are HDS certified (Health Data Hosting) to guarantee the security of patients’ data. Alternatively, you can opt to run our solution on a local system.
- Compatible with standard DICOM imaging format use by MRI vendors (e.g. Siemens, GE Healthcare or Philips)
- We control the quality of all reports through a semi-automated quality control process to verify:
– MRI protocol compliance
– Acquisition artifacts
– Post-processing errors
- Patient reports available as pdf or through our interactive web interface
- Optional PACS integration: to streamline operations, we offer full integration with all common PACS providers. MRI files can be uploaded directly from the IRM console and reports automatically downloaded to the PACS into the patient file. Alternatively, all file transfers can be done through our user-friendly interface.
- Integrated secured messaging system: Users can contact BrainTale support team at any time via the platform, so patient information stays confidential.
- Calibration: Correction of acquisition bias from scanner and center so patients can be compared across centers.
- Standardization: Comparison with data from healthy subjects to establish statistical standards of dispersion measures leading to reliable detection of significant abnormalities in patients.
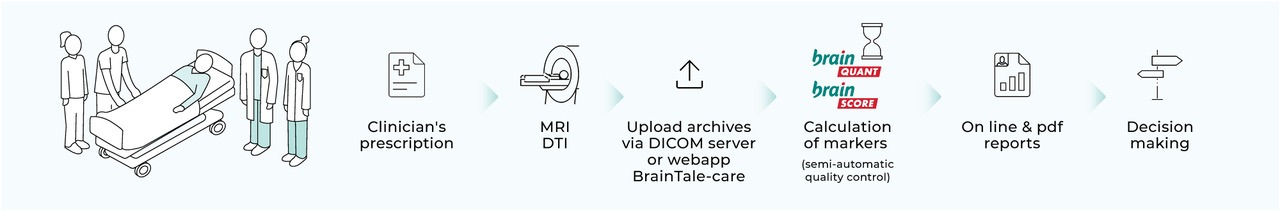
How does it work?
brainTale-care is a software medical device (web platform) to extract standardized parameters from brain Magnetic Resonance Imaging (MRI) and provide prognosis scores for neurological disorders conditions.
brainTale-care provides two analysis modules:
- – brainQuant module for the processing of brain diffusion MR images uploaded on brainTale-care and the provision of the diffusion regional standardized parameters: see the User manual brainQuant module.
- – brainScore-coma module for the provision of a neurologic prediction score related to comatose patient outcome based on diffusion regional standardized parameters from brain diffusion MR images: see the User manual brainScore-coma module.
Clinical warnings
The use of brainTale-care and its modules can by no means replace a diagnosis by a licensed and competent physician, nor constitute medical advice or a diagnosis, which can only be obtained from a physician. The physician bears the sole responsibility for the diagnosis. The calculated parameters are subject to variability and cannot be used without medical advice from a licensed and competent physician.
Legal disclaimers
- BRAINTALE, whose head office is 11 rue de l’Académie 67000 – STRASBOURG, is registered with the Trade and Companies Register under number 840 995 138 RCS STRASBOURG
- Manufacturer address: 140 rue du Chevaleret, 75013 Paris
- BrainTale-care is a Class IIa medical device
- Notified body : BSI Netherlands 2797
- This information deals with brainTale-care platform and modules (brainQuant and brainScore-coma). We invite you to read carefully the instructions for use and the label.
- BrainTale-care is not reimbursed by social security.
- BrainTale is ISO 13485 certified company

Last update : 28/06/2024

