Drug Development and Clinical research
BrainTale provides sensitive, reliable, clinically validated
and easily accessible biomarkers to accelerate CNS drugs development
BrainTale platform contributes actively to the development of new drugs : BrainTale’s biomarkers are developed to be used as relevant endpoints, accelerating and derisking clinical trials as well as supporting treatment efficacy demonstration. The potential of BrainTale biomarkers has already been demonstrated in some demyelinating diseases such as adrenoleukodystrophy (ALD)(1).
At BrainTale, we partner with academic research centers and pharma and biotech companies to develop and deploy relevant biomarkers for CNS diseases, using BrainTale technology allowing the production of calibrated brain white matter measures. To accelerate drug development and ultimately improve patient care.
Brain diseases are the 1st cause of disability worldwide, affecting 1 on 3 people(2). This burden is increasing rapidly with ageing of population. Despite high investment in R&D, neurological diseases remain highly underserved. One reason for drug development failure is the lack of relevant and specific biomarkers to access brain structure. To address this challenge, BrainTale provides sensitive, reliable, clinically validated and easily accessible biomarkers to accelerate CNS drugs development. These biomarkers rely on robust, quantitative and standardized measure of brain white matter based on non-invasive and pain-free data acquisition (diffusion MRI).
Quotes
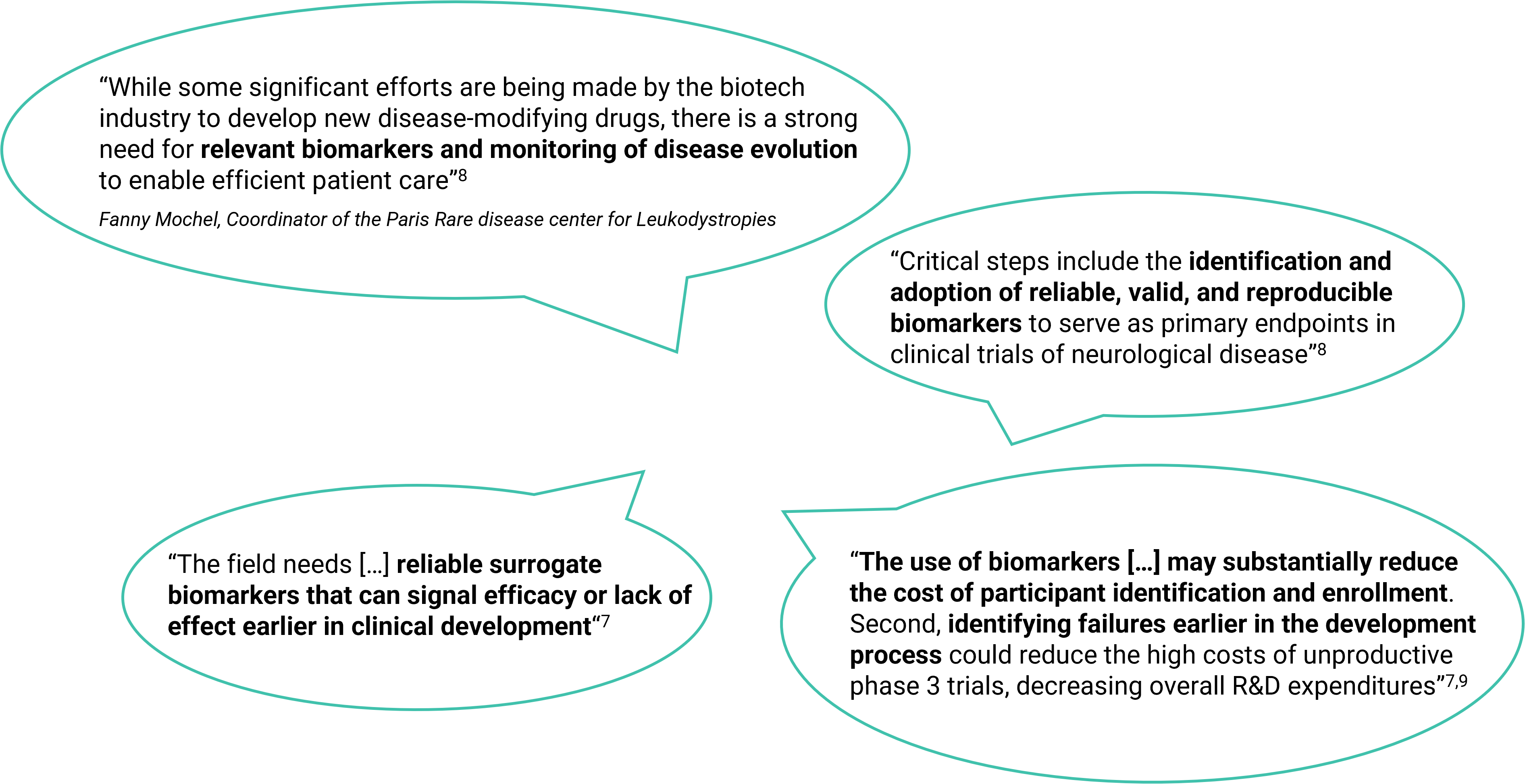
Source :
(1) https://www.braintale.eu/medtech-braintale-gathers-e4-5-million-to-accelerate-the-development-of-its-solution-for-diagnosis-monitoring-and-prediction-of-neurological-disorders/
(2) Feigin, Valery L. “The Evolution of Neuroepidemiology: Marking the 40-Year Anniversary of Publishing Studies on Epidemiology of Neurological Disorders.” Neuroepidemiology 56.1 (2022): 2-3.
(3) Gribkoff V.K., Kaczmarek L.K. (2017) The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology. July 1, 2017
(4) Kaitlin KI (editor). (2014) CNS drugs take longer to develop, have lower success rates, than other drugs. Tufts CSDD Impact Reports. Vol. 16., pp. 1-4. Tufts University, Tufts Center for the Study of Drug Development
(5) Booth B. (2017) Investors venture boldly into neuroscience. Forbes. Sept. 21, 2017, https://www.forbes.com/sites/brucebooth/2017/09/21/venturing-boldly-into-neuroscience/?sh=7201746a3c0e
(6) Cummings, JL, Goldman, DP, Simmons-Stern, NR, Ponton, E. The costs of developing treatments for Alzheimer’s disease: A retrospective exploration. Alzheimer’s Dement. 2022; 18: 469–477. https://doi.org/10.1002/alz.12450
(7) Berger, J.R., Choi, D., Kaminski, H.J., Gordon, M.F., Hurko, O., D’Cruz, O., Pleasure, S.J. and Feldman, E.L. (2013), Importance and hurdles to drug discovery for neurological disease. Ann Neurol., 74: 441-446. https://doi.org/10.1002/ana.23997
(8) Mattke S, Cho SK, Bittner T, et al. Blood-based biomarkers for Alzheimer’s pathology and the diagnostic process for a disease-modifying treatment: projecting the impact on the cost and wait times. Alzheimer’s Dement (Amst). 2020; 12:e12081
(9) Tufts Center for the Study of Drug Development (CSDD) 2014.
BRAINTALE, whose head office is 11 rue de l’Académie 67000 – STRASBOURG, is registered in the Trade and Companies Register under number 840 995 138 RCS STRASBOURG
Last update : 09/07/24

